Prescription Drug Cost Transparency Public Reporting
HCAI collects and publishes prescription drug cost information submitted to the department by drug manufacturers. Manufacturers are required to submit data to HCAI when they implement a Wholesale Acquisition Cost (WAC) increase exceeding 16% over a three year period, as well as when they introduce a new drug to market in California with a WAC that exceeds the Medicare Part D specialty drug cost threshold.
Data is submitted to HCAI by each drug product’s National Drug Code (NDC). For a full list of the specific information collected by HCAI, see the data format and file specifications.
Note: Not all required increases are reported; additionally, some of the reported increases are not required. There are increases that do not meet the statutory requirements for reporting, and there are prescription drugs that do not meet the definition for reporting under the program’s regulations and Section 321(g) of Title 21 of the United States Code. The data reflects only what was reported.
Data Reports
- Wholesale Acquisition Cost Increase Report Data – Current Year
- Wholesale Acquisition Cost Increase Report Data – Cumulative
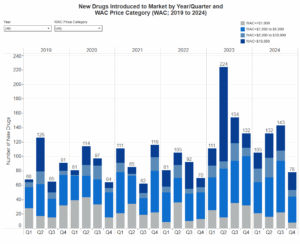
- Prescription Drugs Introduced to Market
Download the Data
WAC Increase and New Drug data submitted to HCAI is updated regularly and is available for download on the Health and Human Services Agency Open Data Portal.
Feedback
HCAI wants your feedback about how you are or are planning to use CTRx data and what you would like to see in the future from the CTRx Program. Share your feedback with HCAI staff by clicking the button below.

CTRx Term Definitions
Wholesale Acquisition Cost
Wholesale Acquisition Cost (WAC) is defined in the U.S. Code as “…the manufacturer’s list price for [a] drug or biological to wholesalers or direct purchasers in the United States, not including prompt pay or other discounts, rebates or reductions in price…”
National Drug Code (NDC)
The pharmaceuticals industry assigns a unique three-segment number, called the NDC, to each drug product manufactured and sold. NDCs must be provided to the federal Food and Drug Administration (FDA), and are used for ordering, billing, inventory management, and recalls.
HCAI requires that relevant reporting uses the 11-digit format of the NDC. The three-segments of the format can be broken into the following:

The labeler code, also known as the manufacturer code, identifies who manufactures, repacks, or distributes a given drug product.
The product code identifies the drug, its strength, dosage form, and formulation specific to the firm that manufactures the given drug product.
The packaging code typically identifies the number of product units in the given product (package size), but also may indicate the type of packaging used.
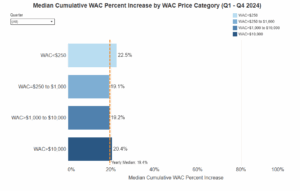
Specified WAC Increases
As specified by California Statute are those “…with a wholesale acquisition cost of more than forty dollars ($40) for a course of therapy … if the increase in the wholesale acquisition cost of a prescription drug is more than 16 percent, including the proposed increase and the cumulative increases that occurred within the previous two calendar years prior to the current year. For purposes of this section, a “course of therapy” is defined as either of the following:
(1) The recommended daily dosage units of a prescription drug pursuant to its prescribing label as approved by the federal Food and Drug Administration for 30 days.
(2) The recommended daily dosage units of a prescription drug pursuant to its prescribing label as approved by the federal Food and Drug Administration for a normal course of treatment that is less than 30 days.
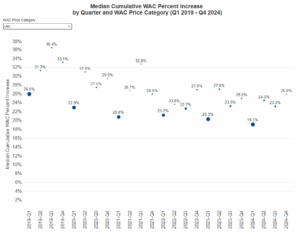
3-Year Percent Increase in WAC
The three-year percent WAC increase is determined by compiling the current year-to-date increase data with the previous two calendar years. If these combined increases exceed a 16 percent price increase, then a WAC increase report must be filed. HCAI calculates its cumulative three-year WAC median percent increase by combining current year-to-date data with the previous two calendar years.
Drug Source
Defined in the U.S. Code as either:
(1) A single source drug which means a drug “…which is produced or distributed under a new drug application approved by the Food and Drug Administration, including a drug product marketed by any cross-licensed producers or distributors operating under the new drug application… and also includes a …drug that is a biological product licensed, produced, or distributed under a biologics license application approved by the Food and Drug Administration…” Prescription drugs of this source type are often referred to as brand drugs.
(2) A multiple source drug which means a drug “…for which there is at least 1 other drug product which— (I) is rated as therapeutically equivalent (under the Food and Drug Administration’s most recent publication of “Approved Drug Products with Therapeutic Equivalence Evaluations”), (II) except as provided in subparagraph (B), is pharmaceutically equivalent and bioequivalent, as defined in subparagraph (C) and as determined by the Food and Drug Administration, and (III) is sold or marketed in the United States during the period.”
(3) An innovator multiple source drug which “…means a multiple source drug that is marketed under a new drug application approved by the Food and Drug Administration…” Prescription drugs of this source type can be primarily referred to as generic drugs, although a manufacturer of this type of generic would have been granted special authorization to produce the generic while the relevant brand drug was still under protection. In addition, this source type includes the brand drug after protection has expired.
(4) A noninnovator multiple source drug which “…means a multiple source drug that is not an innovator multiple source drug.” Prescription drugs of this source type are often referred to as generics.
Annual Dataset Updates
Annual datasets of reports from the preceding year are reviewed in the second half of the current year to identify if any revisions or additions have been made since the original release of the datasets. If revisions or additions have been found, an update of the datasets will be released. Datasets will be clearly marked with ‘Updated’ in their titles for convenient identification. Not all datasets may require an updated release. The review of previously released datasets will only be conducted once to determine if an updated release is necessary. Datasets with revisions or additions that may been made after the one-time review can be requested. These requests should be made via email at ctrx@hcai.ca.gov.